
Rajendra Moorthy, Prateek Shukla and Nabila Fatima, Kemin Industries South Asia Pvt. Ltd., Chennai. India.
Introduction
With adoption of intensive farming practices, the usage of compound feed has increased in dairy industry. Cereal grains and oil cakes are the major ingredients commonly used in a compound feed. These ingredients can be contaminated with fungal organisms during any stage of their growth, harvesting, storage or after processing. Recent changes in the climatic conditions have also favored fungal colonization and mycotoxin contamination of agricultural crops and products. Under tropical conditions, fungal species like Aspergillus, Fusarium and Penicillium are commonly known to infest feed raw materials and produce mycotoxins like aflatoxin B1 (AFB1), ochratoxin, trichothecenes, citrinin, zearalenone, etc.
Aflatoxin: The most notorious mycotoxin
Amongst all mycotoxins, AFB1 is the most extensively studied mycotoxin in dairy cattle, as the excretion of aflatoxin M1 (AFM1) in dairy milk is a public health concern. Regulatory authorities are trying to control the exposure of aflatoxin in cattle and humans through mandating strict limits on aflatoxin levels in feed and milk. However, according to Kemin’s internal survey on AFB1 prevalence, the problem of AFB1 is growing day by day. The survey findings can be summarized as mentioned below:
It was more evident from the prevalence data that, AFB1 is frequently present in the feed and raw materials used for cattle. It is a well-known fact that aflatoxins are potent carcinogens for both cattle as well as human beings. However, various researches also revealed that presence of AFB1 in feed leads to deteriorated feed quality, lower feed palatability and overall lowered animal performance. Acute aflatoxicosis due to consumption of abnormally higher levels of AFB1 causes major signs of liver lesions, leading to congestion and bleeding. Taking cue from these concerns, any incidence of aflatoxicosis in commercial dairy setup can put a serious question mark on the quality and performance of the feed material offered to the animal.
Dietary Mycotoxin Binders: An approach to manage the mycotoxin menace
Various materials, such as activated clays, yeast cell walls, enzymes etc., are being investigated either alone or in combination to manage the problems associated with mycotoxins in dairy animals. Due to the unique structure and physiology of ruminant’s gastrointestinal tract (GIT), it is very essential for any mycotoxin binder to demonstrate its efficacy considering these factors.
Innovation from Kemin
Kemin Industries South Asia Pvt. Ltd. has developed TOXFIN™ XCL, an exclusive formulation for dairy animals in this regard. TOXFIN™ XCL contains mixture of activated clays carefully chosen through unique screening techniques.
In vitro assessment
After careful consideration of different physiological conditions of ruminant’s GIT, Kemin has developed an in vitro triphasic assay to identify the most efficacious ingredient and to test the efficacy of the final product. It was observed that TOXFIN™ XCL showed a high binding of >99% at adsorption step and very less levels of <2% in the desorption step (Figure 1). These results suggest that, the formed AFB1-TOXFIN™ XCL complex would be stable across the entire GIT and helps in eliminating the AFB1 from the body, thereby preventing the formation of AFM1.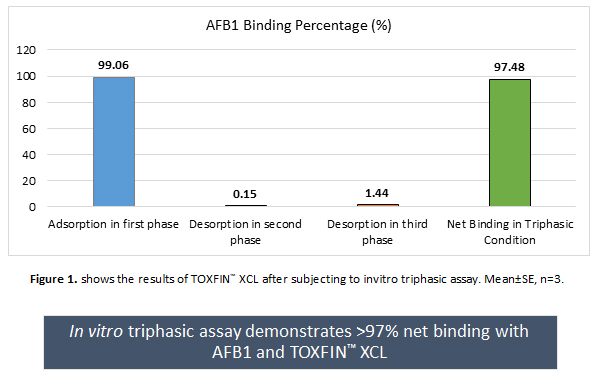
In vivo assessment
To validate the in vitro findings, an in vivo trial was conducted using 20 dairy animals in commercial conditions for 60 days. During the trial period, all the animals were fed with the same diet. The diet was analyzed for AFB1 and the levels were found between 28 and 35 ppb. To evaluate the efficacy of TOXFIN™ XCL and to assess the actual carry-over percentage of AFB1 to AFM1, AFM1 in milk was measured at defined time intervals. The average milk AFM1 level during supplementation of TOXFIN™ XCL was observed to be 0.04 ppb, whereas when no supplementation has been given, the average milk AFM1 level was found to be 0.06 ppb (Figure 2). Interestingly, an increase of more than 40% in milk AFM1 levels have been also observed after discontinuation of TOXFIN™ XCL supplementation.
The maximum residual level (MRL) of AFM1 in milk is 0.05 ppb according to European Food Safety Authority (EFSA). Hence, in this trial supplementation of TOXFIN™ XCL helped to control AFM1 in milk under MRL.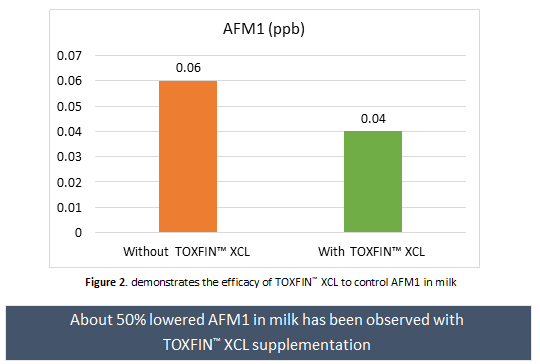
Conclusion
Based on the in vivo and in vitro studies, it can be suggested that TOXFIN™ XCL could able to bind AFB1 inside the animal, thereby preventing the formation of AFM1 and its subsequent secretion into the milk. Moreover, TOXFIN™ XCL can be an efficient choice to manage the mycotoxin menace in dairy animals under commercial conditions.
Note:References available on request.